Animal Cell Membranes Are Primarily Made Up Of Which Two Types Of Lipids?
iii.iii: Lipids
- Page ID
- 1790
Skills to Develop
- Describe the four major types of lipids
- Explain the role of fats in storing energy
- Differentiate between saturated and unsaturated fatty acids
- Describe phospholipids and their role in cells
- Define the basic structure of a steroid and some functions of steroids
- Explain the how cholesterol helps to maintain the fluid nature of the plasma membrane
Lipids include a various grouping of compounds that are largely nonpolar in nature. This is considering they are hydrocarbons that include by and large nonpolar carbon–carbon or carbon–hydrogen bonds. Not-polar molecules are hydrophobic ("water fearing"), or insoluble in h2o. Lipids perform many different functions in a jail cell. Cells store energy for long-term use in the form of fats. Lipids also provide insulation from the environment for plants and animals (Figure \(\PageIndex{1}\)). For example, they aid go along aquatic birds and mammals dry when forming a protective layer over fur or feathers because of their water-repellant hydrophobic nature. Lipids are as well the building blocks of many hormones and are an important constituent of all cellular membranes. Lipids include fats, oils, waxes, phospholipids, and steroids.

Fats and Oils
A fat molecule consists of two main components—glycerol and fatty acids. Glycerol is an organic chemical compound (alcohol) with 3 carbons, five hydrogens, and three hydroxyl (OH) groups. Fatty acids have a long chain of hydrocarbons to which a carboxyl grouping is attached, hence the name "fatty acid." The number of carbons in the fatty acid may range from 4 to 36; almost common are those containing 12–eighteen carbons. In a fat molecule, the fatty acids are attached to each of the three carbons of the glycerol molecule with an ester bail through an oxygen atom (Figure \(\PageIndex{2}\)).

During this ester bond germination, three h2o molecules are released. The three fatty acids in the triacylglycerol may exist like or different. Fats are likewise called triacylglycerols or triglycerides because of their chemical structure. Some fat acids have mutual names that specify their origin. For example, palmitic acrid, a saturated fatty acid, is derived from the palm tree. Arachidic acid is derived from Arachis hypogea, the scientific proper name for groundnuts or peanuts.
Fatty acids may be saturated or unsaturated. In a fatty acid chain, if there are only unmarried bonds between neighboring carbons in the hydrocarbon chain, the fatty acid is said to be saturated. Saturated fat acids are saturated with hydrogen; in other words, the number of hydrogen atoms attached to the carbon skeleton is maximized. Stearic acid is an case of a saturated fat acrid (Effigy \(\PageIndex{iii}\))

When the hydrocarbon chain contains a double bond, the fatty acid is said to be unsaturated. Oleic acid is an example of an unsaturated fat acid (Figure \(\PageIndex{four}\)).

Most unsaturated fats are liquid at room temperature and are called oils. If at that place is one double bond in the molecule, then it is known as a monounsaturated fat (due east.1000., olive oil), and if there is more than than one double bond, then information technology is known every bit a polyunsaturated fat (due east.g., canola oil).
When a fatty acrid has no double bonds, it is known every bit a saturated fatty acid because no more hydrogen may exist added to the carbon atoms of the chain. A fat may contain like or unlike fatty acids attached to glycerol. Long direct fatty acids with unmarried bonds tend to get packed tightly and are solid at room temperature. Animal fats with stearic acid and palmitic acid (common in meat) and the fatty with butyric acid (common in butter) are examples of saturated fats. Mammals store fats in specialized cells called adipocytes, where globules of fat occupy virtually of the cell's volume. In plants, fat or oil is stored in many seeds and is used as a source of energy during seedling development. Unsaturated fats or oils are usually of institute origin and contain cis unsaturated fatty acids. Cis and trans signal the configuration of the molecule around the double bond. If hydrogens are nowadays in the same plane, it is referred to as a cis fat; if the hydrogen atoms are on two different planes, information technology is referred to as a trans fat. The cis double bail causes a bend or a "kink" that prevents the fatty acids from packing tightly, keeping them liquid at room temperature (Figure \(\PageIndex{five}\)). Olive oil, corn oil, canola oil, and cod liver oil are examples of unsaturated fats. Unsaturated fats help to lower blood cholesterol levels whereas saturated fats contribute to plaque formation in the arteries.

Trans Fats
In the food industry, oils are artificially hydrogenated to make them semi-solid and of a consistency desirable for many processed nutrient products. Merely speaking, hydrogen gas is bubbled through oils to solidify them. During this hydrogenation process, double bonds of the cis- conformation in the hydrocarbon chain may be converted to double bonds in the trans- conformation.
Margarine, some types of peanut butter, and shortening are examples of artificially hydrogenated trans fats. Contempo studies have shown that an increment in trans fats in the human diet may lead to an increase in levels of depression-density lipoproteins (LDL), or "bad" cholesterol, which in turn may atomic number 82 to plaque degradation in the arteries, resulting in middle disease. Many fast food restaurants have recently banned the utilize of trans fats, and food labels are required to display the trans fat content.
Omega Fatty Acids
Essential fatty acids are fatty acids required only not synthesized by the human trunk. Consequently, they have to be supplemented through ingestion via the nutrition. Omega-3 fatty acids (like that shown in Effigy \(\PageIndex{6}\)) fall into this category and are one of simply two known for humans (the other existence omega-6 fat acid). These are polyunsaturated fatty acids and are called omega-three because the tertiary carbon from the end of the hydrocarbon chain is continued to its neighboring carbon past a double bond.
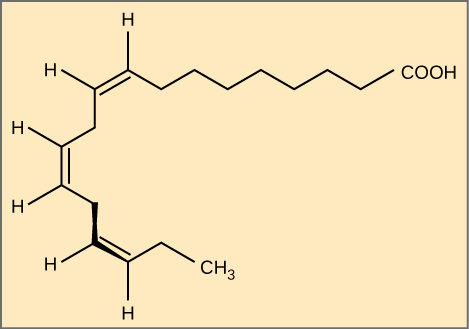
The uttermost carbon abroad from the carboxyl group is numbered every bit the omega (ω) carbon, and if the double bond is between the tertiary and fourth carbon from that end, it is known as an omega-3 fat acid. Nutritionally of import considering the body does not brand them, omega-3 fatty acids include alpha-linoleic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), all of which are polyunsaturated. Salmon, trout, and tuna are skilful sources of omega-3 fatty acids. Inquiry indicates that omega-3 fatty acids reduce the chance of sudden death from center attacks, reduce triglycerides in the blood, lower blood pressure, and prevent thrombosis by inhibiting blood clotting. They also reduce inflammation, and may assist reduce the take chances of some cancers in animals.
Similar carbohydrates, fats have received a lot of bad publicity. It is truthful that eating an excess of fried foods and other "fat" foods leads to weight gain. However, fats practise have important functions. Many vitamins are fat soluble, and fats serve equally a long-term storage form of fatty acids: a source of free energy. They besides provide insulation for the torso. Therefore, "healthy" fats in moderate amounts should exist consumed on a regular basis.
Waxes
Wax covers the feathers of some aquatic birds and the leaf surfaces of some plants. Because of the hydrophobic nature of waxes, they preclude water from sticking on the surface (Figure \(\PageIndex{seven}\)). Waxes are made up of long fatty acid bondage esterified to long-chain alcohols.

Phospholipids
Phospholipids are major constituents of the plasma membrane, the outermost layer of animal cells. Like fats, they are composed of fat acid chains fastened to a glycerol or sphingosine backbone. Instead of three fatty acids attached as in triglycerides, however, there are ii fatty acids forming diacylglycerol, and the tertiary carbon of the glycerol backbone is occupied past a modified phosphate group (Figure \(\PageIndex{8}\)). A phosphate group solitary attached to a diaglycerol does not qualify as a phospholipid; it is phosphatidate (diacylglycerol iii-phosphate), the precursor of phospholipids. The phosphate group is modified past an alcohol. Phosphatidylcholine and phosphatidylserine are two of import phospholipids that are found in plasma membranes.

A phospholipid is an amphipathic molecule, meaning information technology has a hydrophobic and a hydrophilic part. The fat acid chains are hydrophobic and cannot interact with h2o, whereas the phosphate-containing group is hydrophilic and interacts with water (Figure \(\PageIndex{9}\)).

The caput is the hydrophilic part, and the tail contains the hydrophobic fatty acids. In a membrane, a bilayer of phospholipids forms the matrix of the structure, the fatty acid tails of phospholipids face up within, away from h2o, whereas the phosphate group faces the outside, aqueous side (Effigy \(\PageIndex{9}\)).
Phospholipids are responsible for the dynamic nature of the plasma membrane. If a drop of phospholipids is placed in water, it spontaneously forms a construction known as a micelle, where the hydrophilic phosphate heads face up the outside and the fatty acids face the interior of this structure.
Steroids
Different the phospholipids and fats discussed earlier, steroids accept a fused ring structure. Although they do not resemble the other lipids, they are grouped with them considering they are also hydrophobicand insoluble in h2o. All steroids have iv linked carbon rings and several of them, similar cholesterol, take a brusk tail (Figure \(\PageIndex{10}\)). Many steroids also accept the –OH functional group, which puts them in the alcohol classification (sterols).
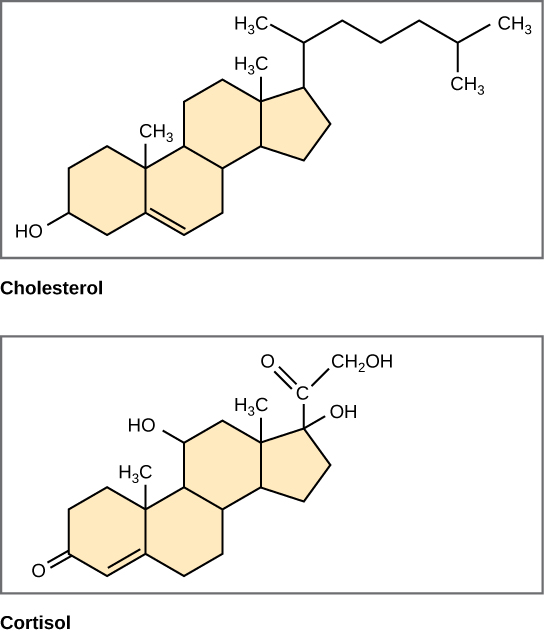
Cholesterol is the near common steroid. Cholesterol is mainly synthesized in the liver and is the precursor to many steroid hormones such as testosterone and estradiol, which are secreted by the gonads and endocrine glands. It is also the precursor to Vitamin D. Cholesterol is also the precursor of bile salts, which help in the emulsification of fats and their subsequent absorption by cells. Although cholesterol is often spoken of in negative terms by lay people, it is necessary for proper functioning of the trunk. Information technology is a component of the plasma membrane of animal cells and is plant within the phospholipid bilayer. Existence the outermost structure in animal cells, the plasma membrane is responsible for the transport of materials and cellular recognition and information technology is involved in prison cell-to-jail cell communication.
Summary
Lipids are a course of macromolecules that are nonpolar and hydrophobic in nature. Major types include fats and oils, waxes, phospholipids, and steroids. Fats are a stored form of energy and are likewise known as triacylglycerols or triglycerides. Fats are made up of fatty acids and either glycerol or sphingosine. Fatty acids may be unsaturated or saturated, depending on the presence or absence of double bonds in the hydrocarbon chain. If only unmarried bonds are nowadays, they are known as saturated fat acids. Unsaturated fat acids may have one or more double bonds in the hydrocarbon chain. Phospholipids brand up the matrix of membranes. They accept a glycerol or sphingosine backbone to which ii fat acid chains and a phosphate-containing group are attached. Steroids are another class of lipids. Their basic structure has four fused carbon rings. Cholesterol is a type of steroid and is an important constituent of the plasma membrane, where it helps to maintain the fluid nature of the membrane. Information technology is also the precursor of steroid hormones such as testosterone.
Glossary
- lipid
- macromolecule that is nonpolar and insoluble in water
- omega fatty
- type of polyunsaturated fat that is required by the body; the numbering of the carbon omega starts from the methyl end or the end that is farthest from the carboxylic end
- phospholipid
- major constituent of the membranes; composed of two fatty acids and a phosphate-containing group attached to a glycerol backbone
- saturated fat acid
- long-concatenation of hydrocarbon with single covalent bonds in the carbon chain; the number of hydrogen atoms attached to the carbon skeleton is maximized
- steroid
- type of lipid equanimous of four fused hydrocarbon rings forming a planar construction
- trans fat
- fatty formed artificially by hydrogenating oils, leading to a unlike arrangement of double bond(s) than those found in naturally occurring lipids
- triacylglycerol (also, triglyceride)
- fat molecule; consists of 3 fatty acids linked to a glycerol molecule
- unsaturated fatty acrid
- long-chain hydrocarbon that has i or more double bonds in the hydrocarbon chain
- wax
- lipid made of a long-chain fatty acid that is esterified to a long-chain alcohol; serves as a protective coating on some feathers, aquatic mammal fur, and leaves
Source: https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(OpenStax)/1%3A_The_Chemistry_of_Life/3%3A_Biological_Macromolecules/3.3%3A_Lipids
Posted by: hinesthestrand.blogspot.com

0 Response to "Animal Cell Membranes Are Primarily Made Up Of Which Two Types Of Lipids?"
Post a Comment